equation for the combustion of methane. Get the update equation for the combustion of methane. Check the bellow calculator with convert equation for the combustion of methane.

Table Of Content:
- Complete Combustion of Methane (CH4) Balanced Equation ...
- Combustion Reaction | CK-12 Foundation
- Balanced Equation for the Combustion of Methane (CH4) - YouTube
- Combustion Reaction ( Read ) | Chemistry | CK-12 Foundation
- A balanced equation for combustion of methane is given below ...
- Methane combustion METHANE CHEMISTRY: the combustion ...
- Combustion reactions
- Write the balanced equation of combustion of methane.
- What is the balanced chemical equation for the combustion of ...
- The balanced chemical equation for the combustion of methane is ...
1. Complete Combustion of Methane (CH4) Balanced Equation ...
https://www.youtube.com/watch?v=_DNF8Z0lMfw Aug 24, 2019 ... CH4 reacts with oxygen (O2) to make carbon dioxide (CO2) and water (H2O). Complete combustion does NOT give carbon monoxide or soot.
Aug 24, 2019 ... CH4 reacts with oxygen (O2) to make carbon dioxide (CO2) and water (H2O). Complete combustion does NOT give carbon monoxide or soot.
2. Combustion Reaction | CK-12 Foundation
https://flexbooks.ck12.org/cbook/ck-12-middle-school-physical-science-flexbook-2.0/section/5.22/primary/lesson/combustion-reactions-ms-ps/
3. Balanced Equation for the Combustion of Methane (CH4) - YouTube
https://www.youtube.com/watch?v=0g73X8vwDSE Mar 27, 2021 ... The reaction for the combustion of methane is: CH4 (g) + O2 (g) = CO2 (g) + H2O (g) The products of CO2 + H2O are typical for combustion ...
Mar 27, 2021 ... The reaction for the combustion of methane is: CH4 (g) + O2 (g) = CO2 (g) + H2O (g) The products of CO2 + H2O are typical for combustion ...
4. Combustion Reaction ( Read ) | Chemistry | CK-12 Foundation
https://www.ck12.org/chemistry/combustion-reaction/lesson/Combustion-Reactions-MS-PS
5. A balanced equation for combustion of methane is given below ...
https://www.toppr.com/ask/en-us/question/a-balanced-equation-for-combustion-of-methane-is-given-belowch4g/ A balanced equation for combustion of methane is given below: CH4(g)+2O2(g)→CO2(g)+2H2O(g) Which of the following statements is not correct on the ...
A balanced equation for combustion of methane is given below: CH4(g)+2O2(g)→CO2(g)+2H2O(g) Which of the following statements is not correct on the ...
6. Methane combustion METHANE CHEMISTRY: the combustion ...
https://torrepiantarosa.com/wp-content/uploads/Combustion.pdf
methane? The simplest chemical reaction involving methane is combustion. ... the chemical equation informs us that any quantity of methane.
7. Combustion reactions
https://web.fscj.edu/Milczanowski/psc/lect/Ch11/slide3.htm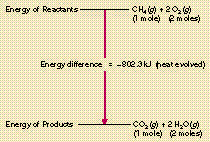 Combustion reactions ; fuel + O · H2O ; The balanced equation for methane is CH4 + 2 O · CO2 + 2 H2O ; 2 C8H18 + 25 O · 16 CO2 + 18 H2O ; CO2 + 2 H2O + energy.
Combustion reactions ; fuel + O · H2O ; The balanced equation for methane is CH4 + 2 O · CO2 + 2 H2O ; 2 C8H18 + 25 O · 16 CO2 + 18 H2O ; CO2 + 2 H2O + energy.
8. Write the balanced equation of combustion of methane.
https://www.toppr.com/ask/en-us/question/write-the-balanced-equation-of-combustion-of-methane/ The balanced equation of combustion of methane is given below: CH4+2O2⟶CO2+2H2O · Write the balanced chemical equation for the following reaction: · Write a ...
The balanced equation of combustion of methane is given below: CH4+2O2⟶CO2+2H2O · Write the balanced chemical equation for the following reaction: · Write a ...
9. What is the balanced chemical equation for the combustion of ...
https://www.wyzant.com/resources/answers/741892/what-is-the-balanced-chemical-equation-for-the-combustion-of-methane
Feb 18, 2020 ... Methane = CH4. Complete combustion of methane: CH4 + 2O2 ==> CO2 + 2H2O. Upvote • 0 Downvote. Add comment.
10. The balanced chemical equation for the combustion of methane is ...
https://study.com/academy/answer/the-balanced-chemical-equation-for-the-combustion-of-methane-is-ch4-g-plus-2o2-g-arrow-co2-g-plus-2h2o-g-which-of-the-following-statements-concerning-this-equation-is-are-correct-1-one-gram-of-ch4-reacts-with-two-grams-of-o2-producing-one-gram-of-c.html
The balanced chemical equation for the combustion of methane is: CH4 (g) + 2O2 (g) → → CO2 (g) + 2H2O (g). Which of the following statements concerning this ...
Conclusion:
Finally, that is all about equation for the combustion of methane. You reached at the last stage of this article. Hope you will get the right information about Complete Combustion of Methane (CH4) Balanced Equation ....
